To understand the importance of protecting thermite welds, we must first identify their location in...
How Cathodic Protection Systems Prevent Pipeline Corrosion

Corrosion or rust (Ferric Oxide) is not an aesthetically pleasing sight. On its surface, its red-brown coloration gives the appearance of a neglected structure in clear disrepair. However, rust's poor aesthetic qualities are obviously the least of our worries. Corroded metal structures (like pipelines) are compromised and in danger of losing mechanical strength.
Rusted structures risk failure as they are susceptible to water permeation and leakage of a stored or transported commodity. For pipes, the risk of bursting keeps us up at night, as holidays (in our industry) are far less fun than the name implies. You may have heard that "cathodic protection systems" work to combat this, but to understand how, we need to look at the molecular processes of rust and protection.
Corrosion and Cathodic Protection
What is corrosion? It is a reaction of a metallic surface with moisture and atmospheric oxygen. The interaction of these three elements causes a specific chemical reaction that results in the metallic or steel structure losing electrons, oxidizing, and rusting. That happens when electrons are lost, but what about when they are gained? you can gain electrons through "reduction." We now find a balance between oxidation and reduction; the movement or flow of electrons is essential to how cathodic protection works.
Metal structures can be protected through a few different barriers, either physical barriers or chemical process barriers. Physical barriers like tapes and protective coatings prevent moisture from infiltration the barrier, thus protecting the metal surface from oxidation. Chemical process barriers offer a more sophisticated approach in the form of Cathodic Protection Systems.
How do Cathodic Protection Systems work?
What is Cathodic Protection? It is a method of protecting metals against corrosion with electric (DC) current. As mentioned above, the loss of electrons eventually causes the steel structure to rust or return to its natural, more stable state.
Cathodic Protection is an electrochemical process that uses a sacrificial metal (the anode) to give up electrons and corrodeᶦ in place of another metal or steel surface (the cathode). You can think of it as a reservoir of spare electrons the system draws from, allowing the primary, functional surface of the structure to be protected.
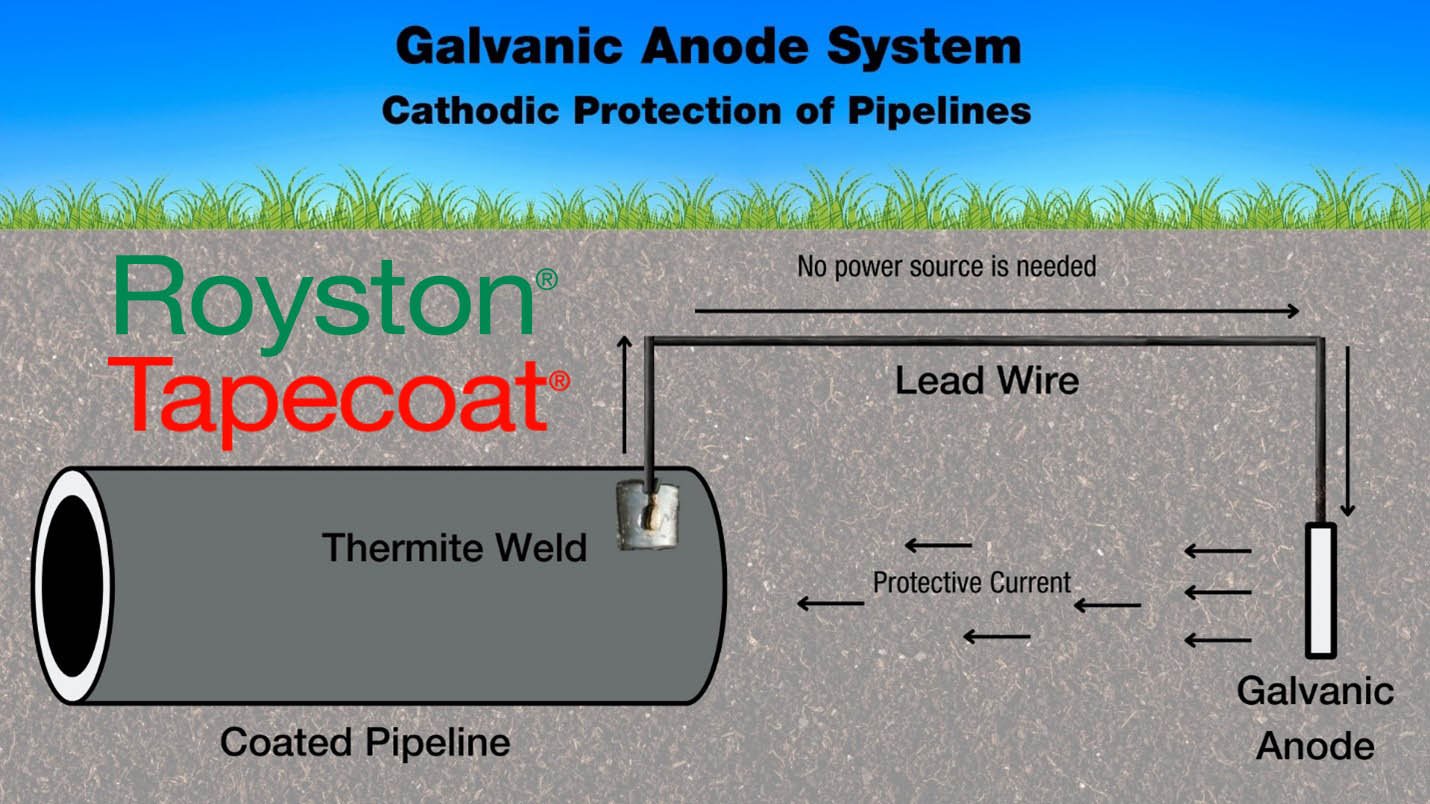
For this to work, there must be an electrolyte. This is typically moisture in the surrounding soil or a body of water. The last item required is a metallic pathway: a cable or wire. The cathode (continually gaining electrons from the anode) remains protected from corroding, whereas the anode (by sacrificing electrons) will corrode in preference to the cathode. This is galvanic cathodic protection.
The other type of cathodic protection employs an external power source to supply the electric current. It effectively "recharges" the electrons in the metal in a conceptually similar way to keeping your laptop plugged in while you use it. As the "battery" drains, it gets more "power" from a source.
The annual cost of corrosion is estimated to be in the trillions of dollars. Some structures in the United States and around the globe that are impacted by corrosion include:
- Steel storage tanks
- Above and underground pipelines
- Steel piers
- Refineries
- Offshore oil platforms
- Wind farms
1: See the Galvanic Series Table








